SKU:
LPK-SVLB
Category: Chemical engineering experimental device
Binary System Vapor – Liquid Equilibrium Data Determination Experiment Device
Through this device, students can understand the significance of determining binary vapor-liquid equilibrium (VLE) data; learn to plot the binary VLE phase diagram; acquire the ability to determine the azeotropic point via the phase diagram of an azeotropic system; and master the method of calculating the activity coefficients of each component using experimentally measured T-P-X-Y data.
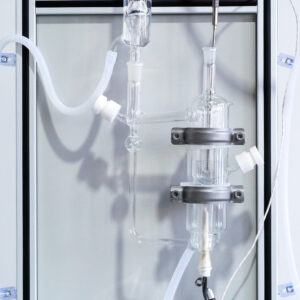
♥ 1. The device can measure vapor-liquid equilibrium (VLE) data of binary systems at atmospheric pressure.
♥ 2. The vapor-liquid equilibrium cell is transparent and visible, allowing observation of experimental phenomena inside the cell.
♥ 3. The equilibrium cell is equipped with a vacuum jacket for insulation. Inside the cell, the liquid and gas phases respectively form independent circulation systems, featuring low sample consumption and rapid equilibrium achievement.
♥ 4. Designed with professionalism, the device enables the acquisition of relevant thermodynamic parameters by analyzing the compositions of the equilibrium vapor and liquid phases, and allows calculation of the activity coefficients of each component using experimentally measured T-P-X-Y data.
♥ 5. Equipment appearance and dimensions: The device is constructed with a high-quality aluminum alloy frame and casters, with overall dimensions no larger than 1480mm×580mm×1780mm (length×width×height).
Product Inquire Now
"*" indicates required fields